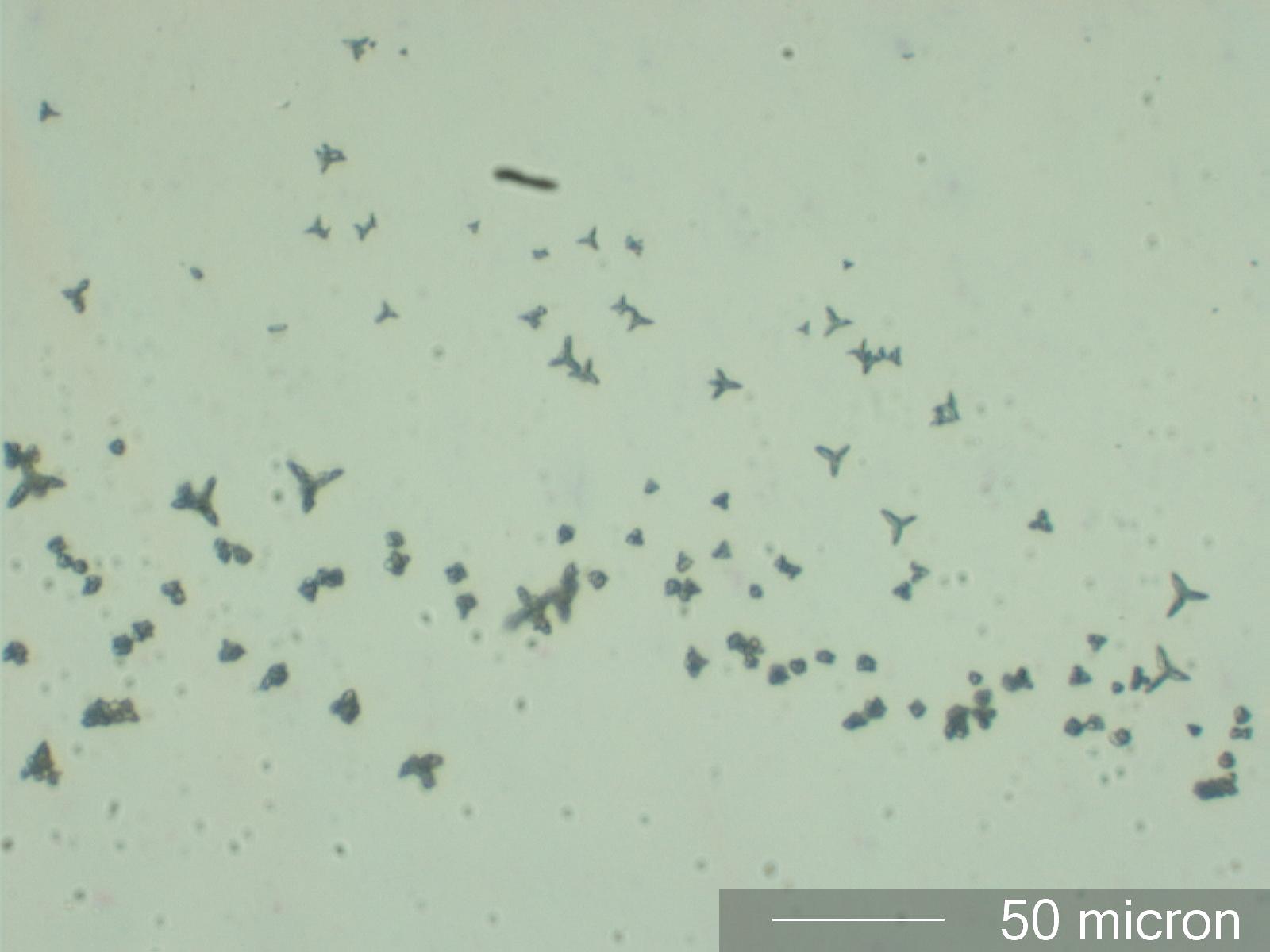
Phosphate with AgNO3
Transmitted Off Crossed Circular Polarized Light Illumination
The presence of phosphate ions in solution can be determined by the addition of a small droplett of 10% silver nitrate solution at the edge of the test solution (See Chamot and Mason, CHEMICAL MICROSCOPY, Vol II, page 317,337-8). This example was created by adding the silver nitrate test solution to a dilute solution of an unknown precipitate removed from a concentated sodium hypochlorite solution and dissolved in dilute nitric acid.
The presence of phosphate ions in solution can be determined by the addition of a small droplett of 10% silver nitrate solution at the edge of the test solution (See Chamot and Mason, CHEMICAL MICROSCOPY, Vol II, page 317,337-8). This example was created by adding the silver nitrate test solution to a dilute solution of an unknown precipitate removed from a concentated sodium hypochlorite solution and dissolved in dilute nitric acid.