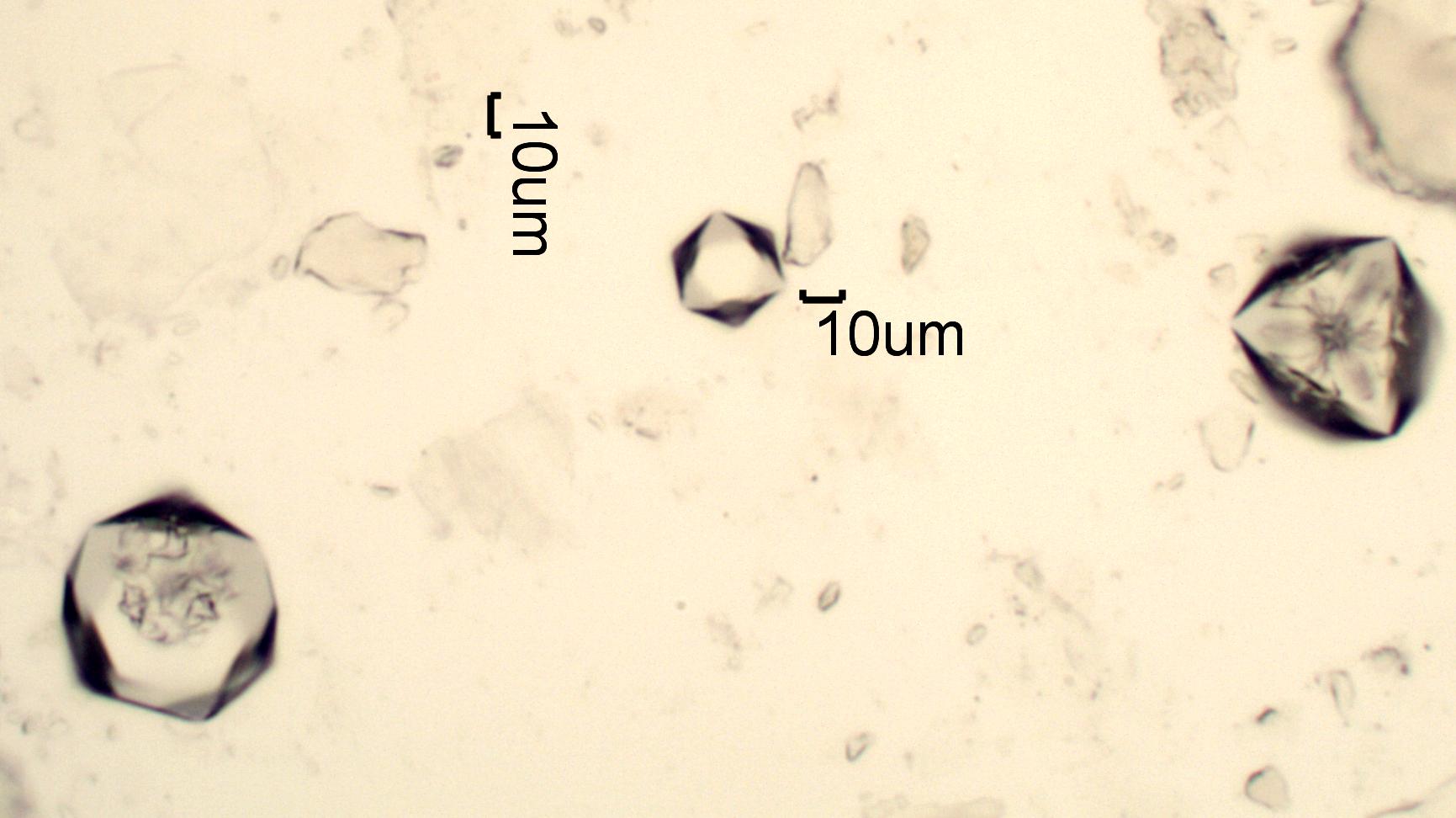
Cesium Alum Crystals
Aluminum in dilute sulfuric acid solution can be detected
with cesium sulfate. These
characteristic isotropic crystals,combinations of cubic and octahedal habits,
indicate the presence of aluminum. This was from a test of a white
powder found on some air handling equipment that had been stored wrapped in plastic
in a covered but uncontrolled environment.
Transmitted Crossed Polarized Light with Red Plate Compensator
Definition/Function:
Aluminum in dilute sulfuric acid solution can be detected with cesium sulfate. These
characteristic isotropic crystals,combinations of cubic and octahedal habits, indicate
the presence of aluminum. This is a standard micro-chemical test from the HANDBOOK OF
CHEMICAL MICROSCOPY, by Chamot and Mason.
Significance in the Environment:
White powders in HVAC condensation pans, around aluminum beams, pipes, etc. are often
aluminum corrosion products, typically Al(OH)3 with
some hydration. If the deposit has aged in the air it may collect carbon dioxide and
form a hydroxide/carbonate complex.
Characteristic Features:
These corrosion products go into solution in dilute sulfuric acid. A few small crystals
of cesium sulfate can be added directly to the solution and
the characteristic crystals will begin forming immediately. The crystals will grow for a
time so letting them sit for ten minutes or so may make
identification easier.
Associated Particles:
If the aluminum was in contact with cement, wallboard, cinder block, or limestone, even
natural minerals in the environment high in calcium then
gypsum crystals will also form. This are birefingent so are easily identified and cannot
be confused with cesium alum.
References:
Chamot, Emile Monnin and Clyde Walter Mason, HANDBOOK OF CHEMICAL MICROSCOPY, Volume II,
pp. 176-8, 1940.