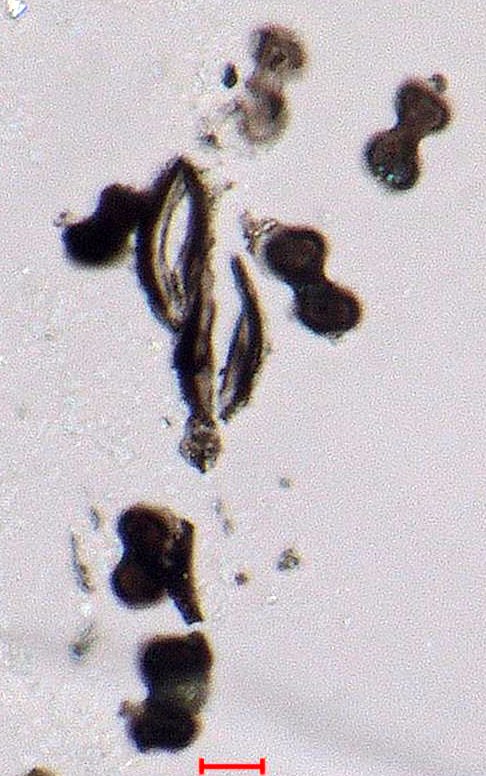
Phytolith from Sugarcane
These are bilobate carbon coated silica phytoliths from sugarcane field burning. Stomata fragments are also present
Transmitted and Reflected Darkfield Illumination
2. Franceschi, Vincent R. and Harry T. Horner Jr., "Calcium oxalate crystals in plants", THE BOTANICAL REVIEW, vol. 46, No. 4, Oct-Dec 1980, pp. 361-427.
3. Piperno, Dolores R., PHYTOLITHS, AltaMira Press, 2006.
4. Rapp, George Jr. and Susan C. Mulholland (eds), PHYTOLITH SYSTEMATICS, Plenum Press, 1992.
5. Madella, M., A. Alexandre, and T Ball, "International Code for Phytolith Nomenclature (ICPN) 1.0", ANNALS OF BOTANY, 2005,available on line at http://aob.oxfordjournals.org/cgi/reprint/mci172v1
6. http://en.wikipedia.org/wiki/Phytoliths
Definition/Function:
Silica phytoliths may become coated with carbon as the surrounding organic material is burned in a low oxygen environment. Reflected darkfield illumination must be used to identify the carbon coating because the interior of these phytoliths can appear black due to light scatter if only transmitted light is used. The light scatter is caused by the formation of an internal froth formed as the phytolith looses moisture due to heat.
Significance in the Environment:
These particles are left behind when plant materials degrade or are burned. The siliceous phytoliths typically become amorphous, transparent particles of distinctive shape. When burned they often become coated with a layer of carbon and appear black or gray. Calcareous phytoliths may remain intact as the plant degrades but they often go through a series of chemical reactions that ultimately result in the formation of a calcium carbonate. When burned they convert to calcium oxide, which then reacts with water and carbon dioxide to form aragonite (calcium carbonate). Cubical calcium oxide and hydroxide particles are common in the plume from the combustion of wood, often showing surface modification to the carbonate. The surface modification is evident as a birefringent film over part of the particle.Characteristic Features:
The silica phytoliths are characterized by their low refractive index, less than 1.48, their cell related morphology (see Madella, Alexandre, and Ball; 2005), and by their lack of birefringence. The other phytoliths are dominated by their crystalline structure rather than cell morphology. The crystalline habit manifested by these phytoliths will vary from plant species to plant species and from cell type to cell type within a species. The two types of calcium oxalate phytoliths from the leaves of Rhubarb are an example of variation between cell types in a single species.Associated Particles:
References:
1. Blinnikov, Mikhail, "Phytoliths in plants and soils of the interior Pacific Northwest, USA", REVIEW OF PALAEOBOTANY & PALYNOLOGY, vol. 135, pp. 71-98, 20052. Franceschi, Vincent R. and Harry T. Horner Jr., "Calcium oxalate crystals in plants", THE BOTANICAL REVIEW, vol. 46, No. 4, Oct-Dec 1980, pp. 361-427.
3. Piperno, Dolores R., PHYTOLITHS, AltaMira Press, 2006.
4. Rapp, George Jr. and Susan C. Mulholland (eds), PHYTOLITH SYSTEMATICS, Plenum Press, 1992.
5. Madella, M., A. Alexandre, and T Ball, "International Code for Phytolith Nomenclature (ICPN) 1.0", ANNALS OF BOTANY, 2005,available on line at http://aob.oxfordjournals.org/cgi/reprint/mci172v1
6. http://en.wikipedia.org/wiki/Phytoliths