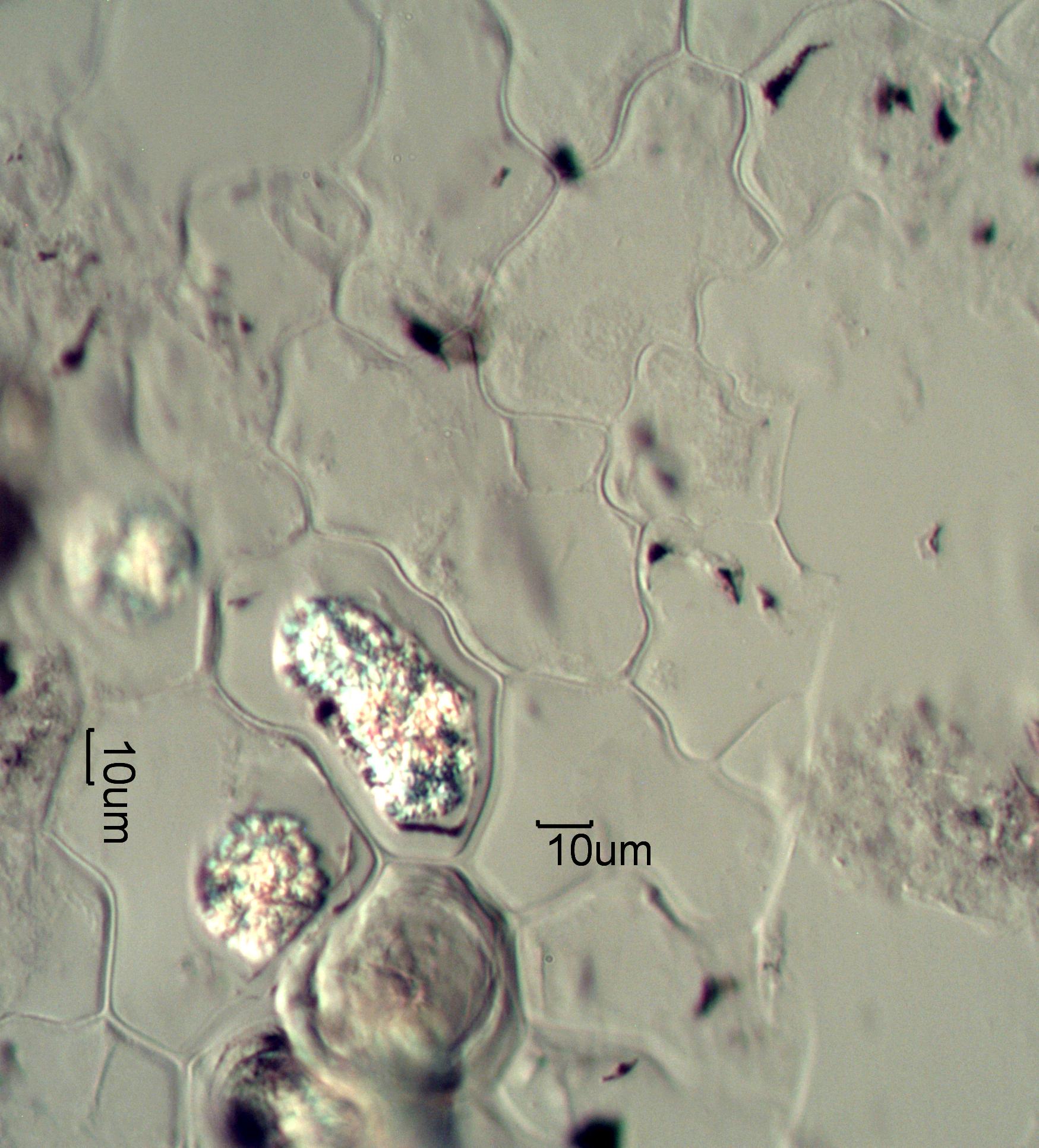
Phytoliths from Ashed Arrowleaf Balsumroot
These are phytoliths from ashed Arrowleaf Balsumroot. This
is from a section of
leaf that was ashed in a muffle furnace. The silica epithelium and a few calcium
oxalate phytoliths are shown here.
Transmitted Off Crossed Polarized Light
Definition/Function:
Significance in the Environment:
Calcareous phytoliths may remain intact as the plant degrades. When the plant containing
calcium oxalate phytoliths is burned
the phytoliths go through a series of chemical reactions. First they begin to loose the
waters of hydration. That begins at about 120 degrees
Celsius. Next, carbon monoxide is released and calcium carbonate begins to form on the
surface of the crystal. That begins at a temperature of about
420 degrees Celsius. At this point the crystal generally still maintains its original
shape. The crystal shows the effect of the exposure to heat
but the shape is still consistent with that characteristic of the original plant.
Continued heating ultimately result in the formation of a calcium
oxide, begining at about 620 Celsius. Cubical calcium oxide and hydroxide particles are
common in the plume from the combustion of wood, often
showing surface modification to the carbonate. The surface modification is evident as a
birefringent film over part of the particle.
Characteristic Features:
Associated Particles:
References: