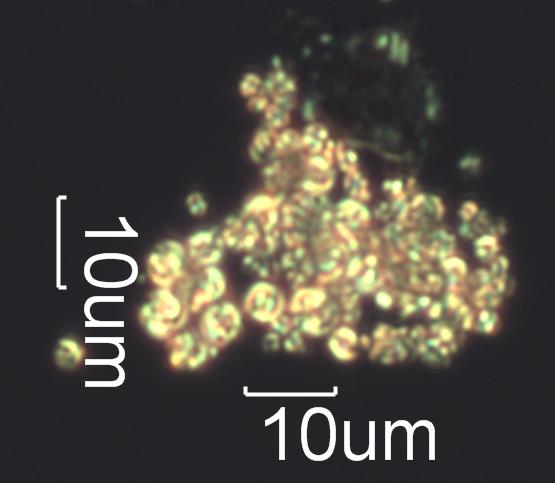
Pyrolyzed Calcium Oxalate Phytoliths
This is from a tapelift from a home impacted by a near
approach of a large forestfire. These particles still
retain the rough form of the original calcium oxalate crystal but the original
crystal has been converted to the spherulite poly-crystalline
form of calcium carbonate.
Transmitted Crossed Circular Polarized Light Illumination
Definition/Function:
Calcium oxalate phytoliths are present in two chemical forms and in many different
crystalline habits in plant material. The two chemical
forms are CaC2O4-H2O, whewellite, and
CaC2O4-H2O, weddellite. Calcium oxalate phytoliths are
exposed to high temperature, water vapor, and carbon dioxide in the plume of a fire.
Calcium oxalate converts
directly to microcrystalline calcite in the temperature range of 430 to 510 degrees
Celsius. If temperatures are higher then calcium oxalate will
convert to calcium oxide and then react with water and carbon dioxide to form calcium
carbonate.
Significance in the Environment:
Characteristic Features:
Weddellite is an optically positive tetragonal crystal with refractive indices of 1.523
(w) and 1.544 (e), for a birefringence of 0.021.
Whewellite is an optically positive monoclinic crystal with refractive indices of 1.490
(a), 1.554 (b), and 1.650 (g), for a birefringence of
0.160. The pyrolysis products of both tend to be calcium carbonate. The calcium
carbonate poly-crystalline aggregate may retain the structure
of the original calcium oxalate crystal but the form of the individual calcium carbonate
crystals making up that shape may be as aragonite or
vaderite tablets or spherulites.
Associated Particles:
References:
http://univ-provence.academia.edu/JacquesBrochier/Papers/198408/Calcite_Crystals_Starch_Grains_Aggregates_or..._POCC_Comment_on_Calcite_Crystals_Inside_Archaeological_Plant_Tissues