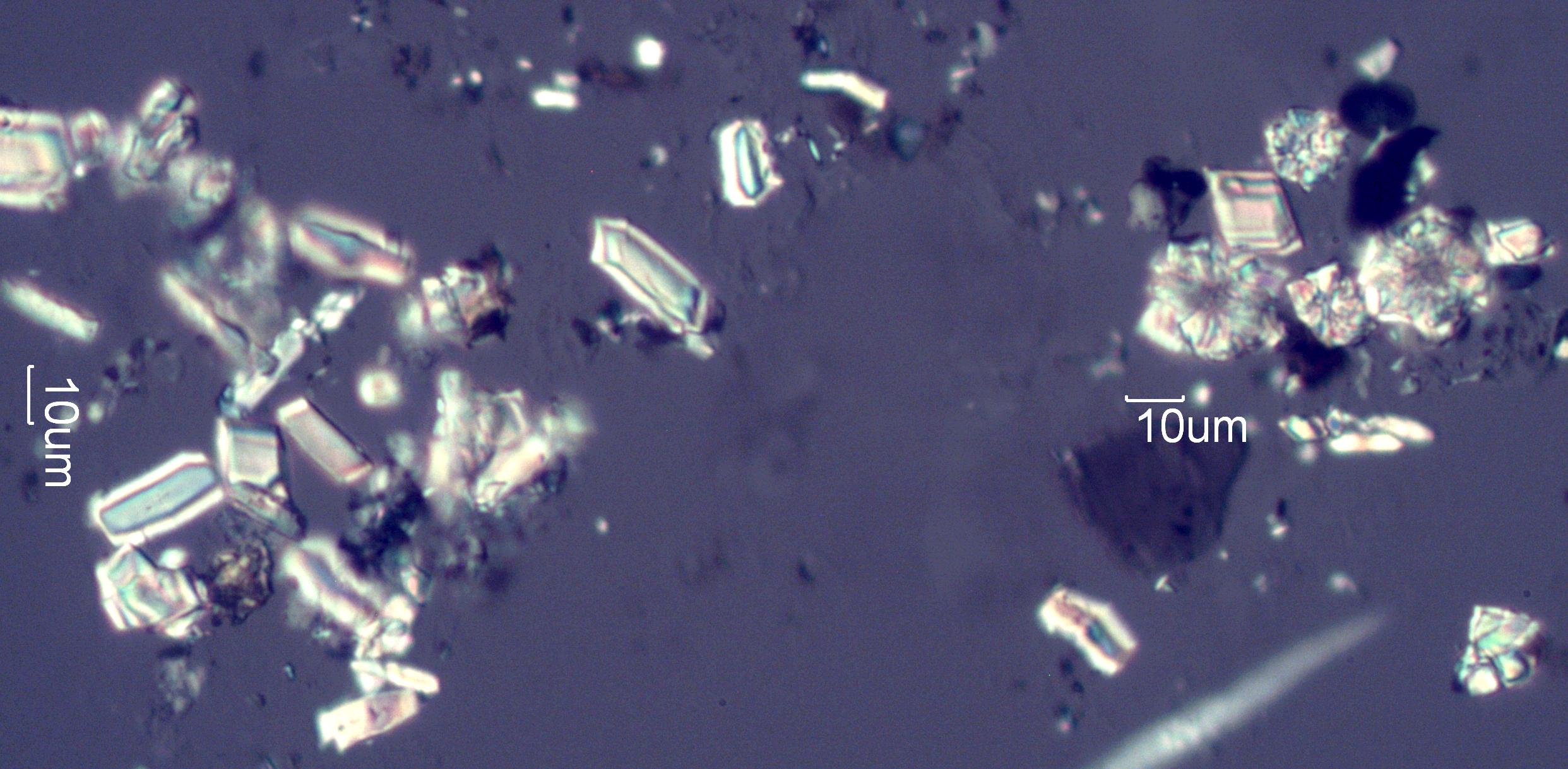
Calcium Oxalate Phytoliths from Hawthorn (Crataegus douglasii) Leaves
This twinned calcium oxalate crystal is from the leaf of a Hawthorn tree, Crataegus douglasii. This shows two of the forms of calcium oxalate phytoliths common in the hawthorn leaf, the twinned (center and left) and the drussy (rossett right). These phytoliths were collected by digesting hawthorn leaves in sodium hypochlorite solution (Bleach).
Transmitted Circular Polarized Light Illumination
2. Franceschi, Vincent R. and Harry T. Horner Jr., "Calcium oxalate crystals in plants", THE BOTANICAL REVIEW, vol. 46, No. 4, Oct-Dec 1980, pp. 361-427.
3. Piperno, Dolores R., PHYTOLITHS, AltaMira Press, 2006.
4. Rapp, George Jr. and Susan C. Mulholland (eds), PHYTOLITH SYSTEMATICS, Plenum Press, 1992.
5. Madella, M., A. Alexandre, and T Ball, "International Code for Phytolith nomenclature 1.0", ANNALS OF BOTANY, 2005,available on line at http://aob.oxfordjournals.org/cgi/reprint/mci172v1
6. http://en.wikipedia.org/wiki/Phytoliths
Definition/Function:
Phytoliths are mineral deposits formed by plant tissue. They may be hydrated silicon dioxide (opal), calcium oxalate monohydrate calcium oxalate dihydrate, calcium phosphate, or calcium carbonate. These structures have distictive shapes and often can help identify the plant of origin when found free in an environmental sample. They are very common airborne particles in arid environments and were identified in the dust captured on the sails of the HMS Beagle in 1833, as reported by Charles Darwin. The calcium oxalate phytoliths from cacti contribute to the calcareous aerosols of the Southwest United States.Significance in the Environment:
These particles are left behind when plant materials degrade or are burned. The siliceous phytoliths typically become amorphous, transparent particles of distinctive shape. When burned they often become coated with a layer of carbon and appear black or gray. Calcareous phytoliths may remain intact as the plant degrades. When the plant containing calcium oxalate phytoliths is burned the phytoliths go through a series of chemical reactions. First they begin to loose the waters of hydration. That begins at about 120 degrees Celsius. Next, carbon monoxide is released and calcium carbonate begins to form on the surface of the crystal. That begins at a temperature of about 420 degrees Celsius. At this point the crystal generally still maintains its original shape. The crystal shows the effect of the exposure to heat but the shape is still consistent with that characteristic of the original plant. Continued heating ultimately result in the formation of a calcium oxide, begining at about 620 Celsius. Cubical calcium oxide and hydroxide particles are common in the plume from the combustion of wood, often showing surface modification to the carbonate. The surface modification is evident as a birefringent film over part of the particle.Characteristic Features:
Associated Particles:
References:
1. Blinnikov, Mikhail, "Phytoliths in plants and soils of the interior Pacific Northwest, USA", REVIEW OF PALAEOBOTANY & PALYNOLOGY, vol. 135, pp. 71-98, 20052. Franceschi, Vincent R. and Harry T. Horner Jr., "Calcium oxalate crystals in plants", THE BOTANICAL REVIEW, vol. 46, No. 4, Oct-Dec 1980, pp. 361-427.
3. Piperno, Dolores R., PHYTOLITHS, AltaMira Press, 2006.
4. Rapp, George Jr. and Susan C. Mulholland (eds), PHYTOLITH SYSTEMATICS, Plenum Press, 1992.
5. Madella, M., A. Alexandre, and T Ball, "International Code for Phytolith nomenclature 1.0", ANNALS OF BOTANY, 2005,available on line at http://aob.oxfordjournals.org/cgi/reprint/mci172v1
6. http://en.wikipedia.org/wiki/Phytoliths