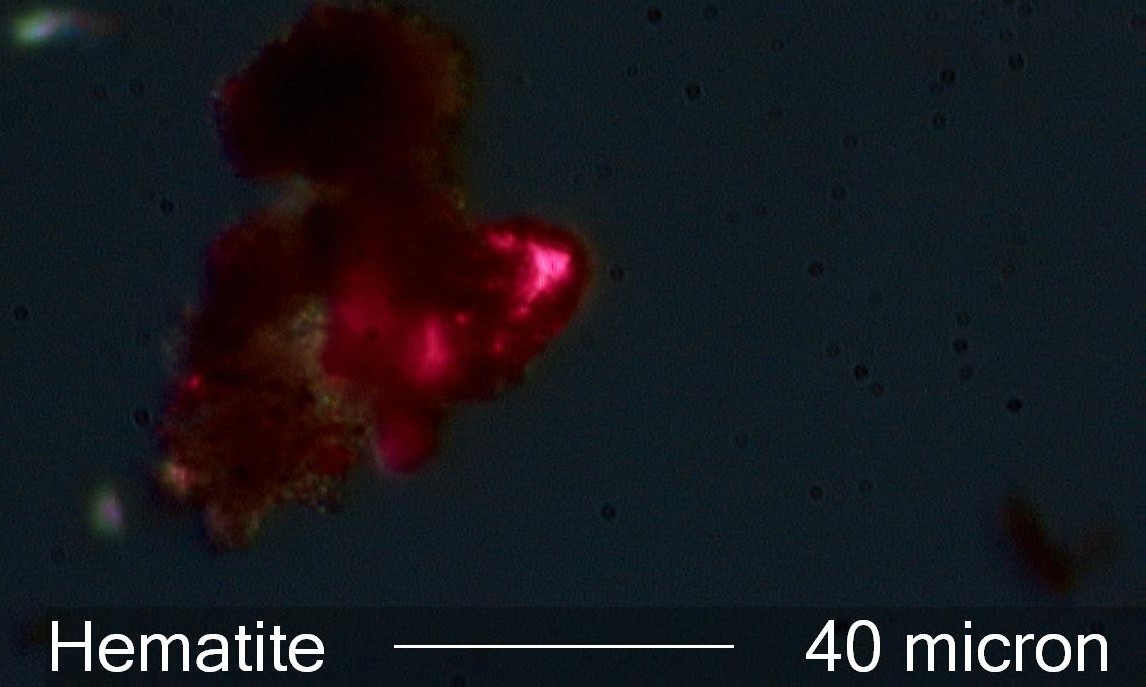
Hematite
This is a single rather large crystal of hematite. This
size tends to be indicative of a natural mineral rather
than a rapidly formed hematite. This crystal has a tabular form so will still
transmit some red light as the polarizing filters are rotated
from the crossed position. The clay associated with the particle adds to the
structure that implys a natural source rathter than an anthropogenic
source. This particle was from the surface of a flash-over failed bushing due to
metal work that had been done in the sub-station yard. Hematite
is not always indicative of human activity.
Transmitted Crossed Circular Polarized Light
Definition/Function:
Hematite is Fe2O3. It is the fully oxidized form of iron. Hematite
is a natural mineral but can also be the endpoint of
iron corrosion or the result of burning iron. The sparks that come from abrasive
grinding of iron are burning fragments of iron that result in
small spheres of hematite.
Significance in the Environment:
Hematite spheres are a good indication of abrasive grinding of iron but may also be
produced by the spark from a lighter. Welding or torch cutting
of iron or iron alloys tends to produce magnetite spheres but may produce some rare
hematite spheres. The hematite spheres tend to be closer
to the source than the magnetite spheres because the magnetite spheres tend to be
balloons that travel much farther (see magnetite spheres).
Hematite spheres may also be formed by burning powdered coal but more often the iron
minerals in the coal color the glass produced as flyash.
Hematite that is the end product of iron corrosion tends to be polycrystalline with the
individual crystals rarely larger than about 2 micrometers
in diameter.
Natural hematite tends to form larger crystals and can be identified as natural
background by this feature. Smaller crystal size doesn't exclude
a natural source but its natural origin will be indicated by the presence of typical
associates rather than crystal size.
Characteristic Features:
Hematite has a refractive indices of about 2.9 for epsilon and 3.2 for omega for a
birefringence of about 0.3. Crystallographically it belongs
to the trigonal subclass of the hexagonal crystal system. It is essentially opaque to
blue light and only transmits a small amount in red light.
As a result it will often appear opaque with brightfield illumination but will show as
blood red in crossed polarized light. It has a reflectivity
of about 30% and appears red with reflected darkfield illumination. In polished sections
viewed with reflected brightfield illumination it often
shows deep red internal reflections.
Associated Particles:
Hematite spheres will be associated with cigarette ash, charred leaf, or other charred
plant material if from a lighter. Hematite spheres will
be associated with other glassy flyash if from a combustion source. They will be
associated with abrasives such as emery, silicon carbide, or
even diamond and abraded metal if the source is metal grinding.
Hematite will always be associated with limonite, goethite, and sub-oxides if the source
is iron corrosion.
References:
Deer, W. A., R. A. Howie, and J. Zussman, AN INTRODCUTION TO THE ROCK-FORMING MINERALS,
ISBN 0-582-30094-0, pp. 540-542, 1992.