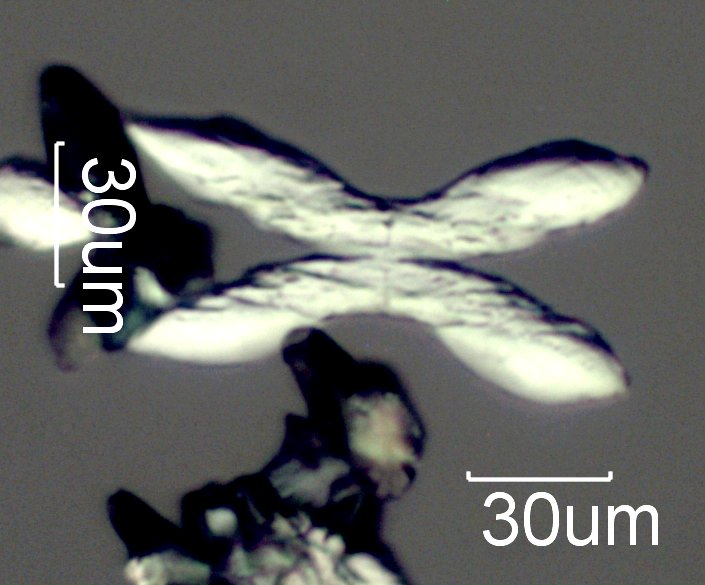
Ammonium Magnesium Phosphate Crystals
Radiating stars and crosses form first, such as the one shown here. A little later rectangular crystals with a "trussed roof" structure appear.
Transmitted Oblique Off Crossed Circular Polarized Light
Definition/Function:
This is a standard micro-chemical test for magnesium using sodium phosphate in an ammoniacal solution from the HANDBOOK OF CHEMICAL MICROSCOPY, Vol 2, by Chamot and Mason, pages 130-131. The product formed is NH4MgPO4-6H2O (numbers are subscripts not superscripts).