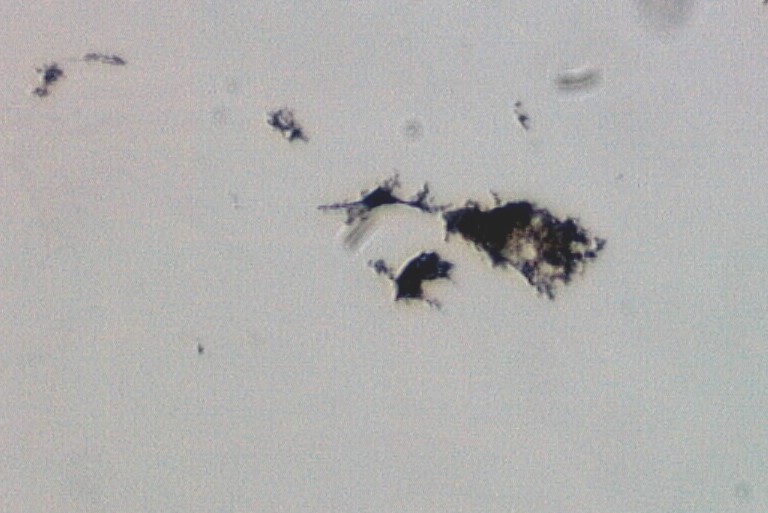
Candle Soot
This is an example of the agglomerated soot particles
typical of candle soot. This is a taplift
sample from a home that burned a large number of candles on a regular basis.
Transmitted Off Crossed Circular Polarized Light
Definition/Function:
Candle soot consists of particles from a few nanometers in diameter to agglomerates of
many micrometers or even tens of
micrometers in diameter. The larger agglomerates tend to be rich in partially pyrolyzed
wax. The formation of these
agglomerates is largely the result of convection and turbulent air flow around the wick.
The flame is the result of burning
wax vapors, the result of melting the wax, drawing the wax up the wick by capillary
action, boiling the wax from the wick,
and then combusting the wax in the flame. The high molecular weight of the wax results
in a high oxygen demand during
combustion. The oxygen in the air is quickly consumed by the hydrogen from the wax
molecule (wax is composed of carbon and
hydrogen in basically a ratio of two hydrogen to each carbon) and any residual oxygen is
then available to combine with the
carbon to form carbon dioxide. Providing more air tends to rush the wax vapor and the
unconsumed carbon residues in the
flame to the cooler environment of the room away from the flame. As a result the wax
condenses around the carbon particles
(soot) and the particles stick together to form large agglomerates, as in this
photograph. If candles are burned in a
home the chemical signature of the wax will often dominate the results of a mass
spectrometer analysis even if candle soot
is not the major source of soot in the home. Many other sources of soot produce
relatively little trace of their source in
residual chemicals identifiable by mass spectrometry. Light microscopy plays an
essential role in the characterization of
soot in homes.
Significance in the Environment:
The combustion of candles in an indoor environment invariably results in the deposition
of soot on surfaces. Some of that
soot is present in the form of agglomerated particles with a high hydrocarbon content.
The hydrocarbon is a solid at normal
room temperatures which results in the irregular form of the agglomerates. These types
of particles may be produced by the
burning of thermoplastics, heavy oils, tar, asphalt, etc. Fires in buildings result in
the formation of similar appearing
particles due to the combustion of materials used in the construction of the building or
furniture in the building. Many
other types of combustion particles are created by such fires and these other particles
help to properly apportion the
relative contribution of soot from various sources.
Characteristic Features:
Candle soot agglomerates are irregular in outline, opaque, highly light absorbing with
no highlights when viewed with
reflected darkfield illumination, and have a very irregular feathered edge.
Associated Particles:
References: