|
Physiochemical Properties
Physiochemical properties are features evident as a result of chemical
composition, crystallography, or
special arangment of molecules, ions, electron bond features, or excitation
effects that can be made visible. Illumination systems include polarized
light,
phase contrast, interference systems, fluorescence, etc. The example
immediately
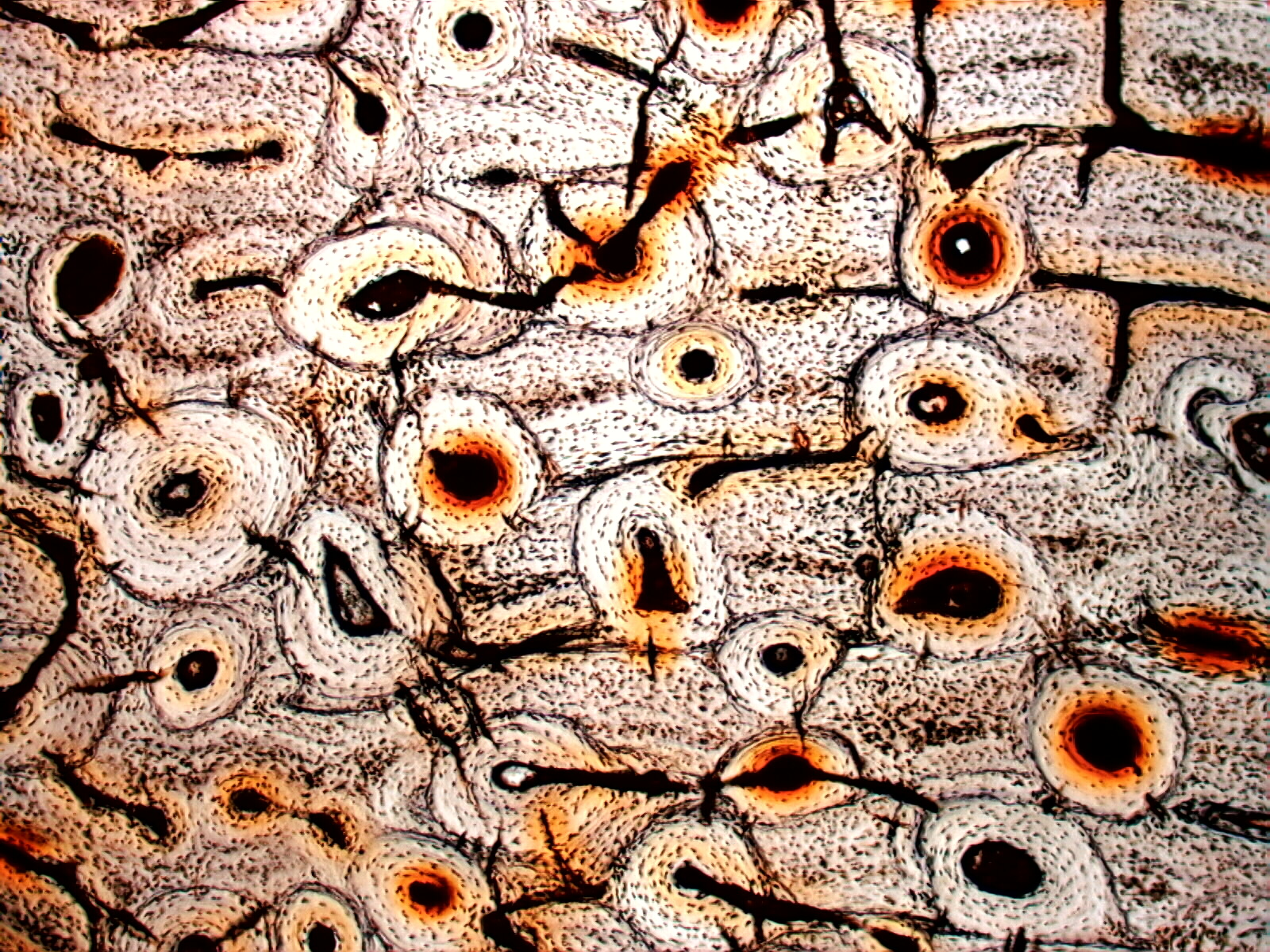
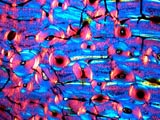
below is a thin section of dinosaur bone. The first image shows the
structure
and color of the fossil bone. The second image is the same view showing the
crystallographic orientation of the minerals making up the fossil by using a
red plate compensator. These are just a few examples of the feature that are
part of this section.
Refractive Index
The refractive index of a particle is a fundamental property of the particle
and is intimately associated with the mechanical
properties of the material. it is the result of the electron density per
unit volume and the nature of the molecular and
crystalline bonds holding the material together. With the particle in a
fixed mounting medium the ability to measure its
refractive index as a function of wavelength is limited to the Duc de
Chaulnes method. More information on the particles
refractive index may be available from the effects at the interface between
the particle and the mounting medium. That is
covered in the section on "Interface Properties".
Transmission
The color and brightness of a particle viewed with transmitted light is the
result of the electron density and bond energies
of the molecules that make up the particle. Morphology caused light scatter
has been discussed above and affects the light that
is transmitted. Here we are considering the net effect of scatter,
reflection, and absorption. Surface scatter and reflection
are affected by the medium around the particle. In a fixed mounting medium,
particles will behave in a fixed way

Absorption
Absorption is the light lost in the particle that is converted from
electromagnetic energy into thermal energy. Absorption
is often wavelength dependent and contributes to the apparent color of the
particle.
. . . Hemetite
Hematite has a complex refractive index of 2.937 + i(0.24268) for epsilon.
The omega refractive index is about 3.2 + i(0.1) at the same wavelength.
When using transmitted light hematite appears nearly opaque. With its high
birefringence, approximately 0.28, even small particles appear red between
crossed polarizing filters. It appears red because it transmits red
wavelengths much more efficiently than the shorter wavelengths (blue, green,
yellow).
With Brightfield illumination the background is too bright to see the small
amount of red light transmitted. With crossed polarizers the
background is dark and the light transmitted as a result of the high
birefringence of hematite and its absorption properties is red.




. . . Tourmaline
Polished sections of tourmaline were the first linear polarizing filters.

Reflectivity
The reflectivity of the particle is evident in reflected light. It is a
result of the electron density difference between the
particle and the mounting medium and the electrical conductivity and
magnetic permeability of the molecules that make up the particle.
Transmission Color
The transmitted color of the particle consists of the wavelengths that are
least affected by reflection and absorption.

Reflected Color
The reflected color is the result of the wavelengths at which the refractive
indices of the mounting medium and the particle are
most different and the loss of the wavelengths that are absorbed.
Birefringence
Birefringence is the property of showing more than one refractive index as a
function of particle orientation and wavelength.
Such a particle will exhibit interference colors when viewed between crossed
circular polarized filters. They will typically show
extinction positions (see below) with rotation of the stage when viewed
between crossed linear polarizing filters.
. . . Low Birefringence (20 to 100 micrometer thick particles don't
exceed yellow)

. . . Moderate Birefringence (20 micrometer thick particles show yellow
to blue)

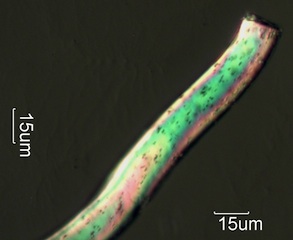
. . . High Birefringence (20 micrometer thick particles exceed blue, 1
micrometer particles are white)


. . . Anomalous Birefringence (colors are abnormal)
Anomalous birefringence is the result of birefringence varying by wavelength.
The result is anomalous interference colors. Silicon
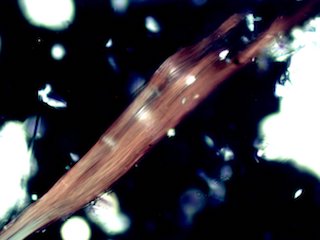
carbide and crocidolite asbestos are two common examples. Crocidolite has
higher birefringence in red light (longer Wavelengths) than in
blue. As a result, very thin fibers of crocidolite appear red between
crossed polarizing filters. Thicker fibers appear blue because of
the strong blue color of the mineral.

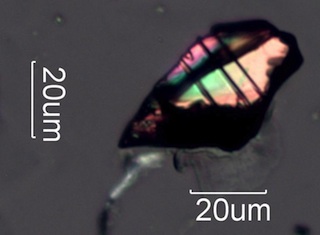
Silicon carbide has higher birefringence in blue light (Shorter Wavelenghts)
than in red. As a result, blue wavelengths cycle more
rapidly than red wavelengths. Yellow interference color begins for thinner
particles and first order red appears purple because blue
is increasing well before red significantly decreases. This effect changes
the color sequence through the whole range of microscopic
silicon carbide particles.

. . . Stress Birefringence
When a material is placed under stress the distribution of the electrons in
the material is changed. The amount of change is different for each
material and is a characteristic of the material. The photoelastic constant
of the matrial is a measure of the electron displacement (strain) as a
function of the load (stress) applied as long as the deformation is elastic,
springs back when the load is removed. If the Young's Modulus of the
matrial is exceeded, then some of the deformation becomes permenant. In some
materials the applied load can be "frozen" in place, as in the case of
high stress glass sheet. Polarized light can make the displacement visible.
Both plastic deformation and elastic deformation result in an
anisotropic distribution of electrons in the material that becomes visible
as interference colors when the object is viewed between crossed linear or
crossed circular polarizing filters. Click on the photographs below for more
information.
. . . . . . Stress Birefringence in Skin Cells


. . . . . . Stress Birefringence in Safety Glass

. . . Conductivity Birefringence (Hall Effect?)
Polarized light is depolarized at the interface between a conductive particle
and a non-conductive mounting medium. This light halo effect with
transmitted crossed polarized light indicates an opaque particle is a wear
metal particle or at least is conductive. Graphite is sufficiently
conductive
to produce this effect. Pencil debris can be distinguished from combustion
residue by this effect.
. . . . . . Fretting Metal Wear



. . . . . . Graphite



. . . . . . Magnetite Spheres

. . . Form Birefringence
. . . False Birefringence
If the refractive index of a transparent particle is much different than the
medium in contact with it, then the polarized beam can be
changed at the interface as a result of reflection. If the interface is
aligned with the polarizer or analyzer then the beam is not
affected. In other orientations reflection at the interface results in
rotation of the polarized beam and the interface appears to show
a first order white interference color.

Bireflection
Bireflection is the property of having different reflectivities in different
directions of linear polarized light. It is most
evident when viewed with reflected crossed polarized light. The object
appears bright on a black background.
Pleochroism
Pleochrism is the property of changing color on rotation when viewed with
linear polarized light. Many colored materials show this property.
. . . Crocidolite

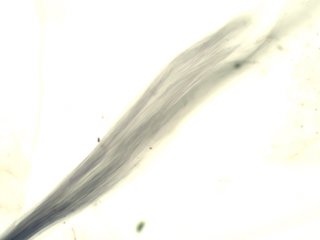
. . . Hornblende


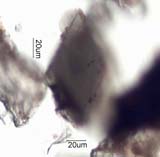
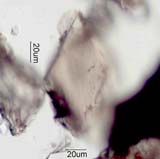
. . . Tourmaline

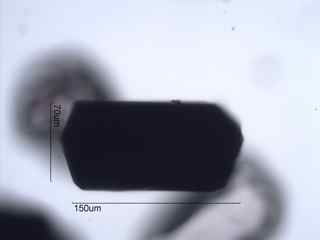




. . . Olivine



Optical Sign
The optical sign of a birefringent or bireflective particle is either
positive or negative depending on the relative value of its
refractive indices.
Sign of Elongation
The sign of elongation is positive or negative depending on the orientation
of its highest refractive index to the long axis of the
partical.
Interference Colors
Interference colors may result from birefringence or from thin film effects.
. . . Thin Film Interference Colors



. . . Birefringence Interference Colors



Extinction
As an anisotropic particle between crossed linear polarizing filters is
rotated in the plane of the stage by rotating the stage it
generally goes dark every 90 degrees, but not always. If the particle goes
dark (extincts) and has a characteristic morphology then its
"extinction position" can be said to be parallel to a characteristic
morphology (parallel extiction), bisect a characteristic angle
(symmetric extinction), or, if neither of those conditions are satisfied,
then it has oblique extinction. Some anisotropic materials
don't change on rotation of the stage. Mature cotton fibers are an example.
They have no extiction position. Other materials my darken
but not go extinct. Peristerites are an example. Some materials shade into
reddish, then bluish darker positions on rotation because
the extinction position for red and blue wavelengths are not aligned. Wood
fibers are a common example.
. . . Parallel Extinction
. . . Symmetric Extinction
. . . Oblique Extinction
. . . Partial Extinction
. . . No Extinction
interference Pattern
. . . Biaxial Interference Pattern
. . . Uniaxial Interference Pattern
. . . Ambiquous Interference Patterns
Twinning
Twinning in a crystal is the result of a change in crystallograph orientation
along a plane. It may be evident by the crystal having
different extinction position along different planes, as with the feldspar
crystal below, or by showing alternating pleochroism, as with
the olivine crystal below.



Fluorescence

|